DEPARTMENT OF PHARMACY PRACTICE
About Department
The Department of
Pharmacy Practice was established in the year 2014, offers Pharm D with an Intake of
30. The department provides spacious and well equipped laboratories with the latest
infrastructure. The department having well experienced competent faculties.
Placements are being provided for Pharm. D students in MNC and Corporate
Hospitals like Novo Nordisk India Private Limited , Altruist Technologies Pvt Ltd ,
Pace Hospital , Yashoda Hospital etc .
The Department of Pharmacy Practice is mainly concentrating on social
welfare activities by conducting and participating in various programs like Medical
camps, Blood donation camps, Medical awareness programs, Service Programs, Rallies
etc.
VISION AND MISSION
- AIPS vowed to promote an academic setting that encourages students' Future-Directed growth in practice, research, education, and scholarly projects, as well as to help students become professional pharmacists for clinical research in hospitals and communities.
Our Vision
- To develop highly skilled, patient-focused professionals with excellent moral principles who can adapt to the ever-changing needs of the healthcare industry.
Our Mission
COs - PG
| DEPARTMENT OF PHARMACY PRACTICE COURSE OUTCOMES | |||
| S.NO | Year/Sem | Course Name | Course Outcomes |
|---|---|---|---|
| 1 | I | HUMAN ANATOMY AND PHYSIOLOGY | CO1: They would have learnt the gross anatomy, histology and physiology of various organs of the human body. |
| CO2: They would identify the various tissues and organs associated with the different organ systems with help of charts and specimens. | |||
| CO3: They would have studied the coordination in functioning of different organs of each system. | |||
| C04: They would have understood the several physiological homeostatic mechanisms and their imbalances in human body. | |||
| 2 | I | PHARMACEUTICS | CO1: Upon completion of this program the student will know the formulation aspects of different dosage forms. |
| CO2: Do different pharmaceutical calculation involved in formulation | |||
| CO3:Formulate different types of dosage forms | |||
| CO4:Appreciate the importance of good formulation for effectiveness. | |||
| 3 | I | MEDICINAL BIOCHEMISTRY | CO1: To understand the importance of metabolism of substrates. |
| CO2: Will acquire chemistry and biological importance of biological macromolecules. | |||
| CO3: To acquire knowledge in qualitative and quantitative estimation of the biological macromolecules. | |||
| C04: To know the interpretation of data emanating from a Clinical Test Lab. | |||
| 4 | I | PHARMACEUTICAL ORGANIC CHEMISTRY | CO1: To be able to give systematic names to simple organic compounds and poly functional group. |
| CO2: To achieve an understanding of the behavior of organic compounds and to establish a foundation for studies into natural and synthetic products of pharmaceutical interest. | |||
| CO3: To acquire the knowledge and understanding of the basic experimental principles of pharmaceutical organic chemistry. | |||
| C04: To draw the structures and synthesize simple pharmaceutically active organic compounds. | |||
| 5 | I | PHARMACEUTICAL INORGANIC CHEMISTRY | CO1: Well acquainted with the principles of limit tests |
| CO2: Understand the principles and procedures of analysis of drugs and also regarding the application of inorganic pharmaceutical | |||
| CO3: Knowledge about the sources of impurities and methods to determine the impurities in inorganic drugs and pharmaceuticals | |||
| C04: Appreciate the importance of inorganic pharmaceuticals in preventing and curing the disease. | |||
| 6 | I | REMEDIAL MATHEMATICS | CO1: Apply mathematical concepts and principles to perform computations for Pharmaceutical Sciences. |
| CO2: Create, use and analyze mathematical representations and mathematical relationships | |||
| CO3: Communicate mathematical knowledge and understanding to help in the field of Clinical Pharmacy | |||
| C04: Perform abstract mathematical reasoning | |||
| S.NO | Year/Sem | Course Name | Course Outcomes |
|---|---|---|---|
| 1 | II | PATHOPHYSIOLOGY | CO1: Describe the etiology of the selected disease states. |
| CO2: Describe the Pathogenesis of the selected diseased states. | |||
| CO3: Name the signs and symptoms of the diseases. | |||
| CO4: Mention the complications of the diseases | |||
| 2 | II | MICROBIOLGY | CO1: To study the apparatus used in microbiology & preparation, sterilization of glassware’s and media |
| CO2: To study different staining techniques, motility characters, enumeration of microorganism, method of isolation of pure culture and biochemical testing for identification of microorganism | |||
| CO3:To perform culture sensitivity testing, sterility testing for powder & liquid and determination of MIC | |||
| CO4: To perform microbiological assay of antibiotics, vitamins and determination of RWC, Widal, Malaria parasite | |||
| 3 | II | PHARMACOLOGY-I | CO1: Understand the pharmacological aspects of drugs falling under the above mentioned chapters. |
| CO2: Handle and carry out the animal experiments. Correlate and apply the knowledge therapeutically. | |||
| CO3: Appreciate the importance of pharmacology subject as basis of therapeutics. | |||
| 4 | II | PHARMACOTHERAPEUTICS- I | CO1: The Pathophysiology of selected disease states and the rationale for drug therapy. And therapeutic approach to management of these diseases. |
| CO2:. Describe the Pathophysiology of selected disease states and explain the rationale for drug therapy. | |||
| CO3: The controversies in drug therapy; The importance of preparation of individualised therapeutic plans based on diagnosis; Needs to identify the patient-specific parameters relevant in initiating drug therapy and monitoring therapy. | |||
| CO4: Summarise the therapeutic approach to management of these diseases including reference to the latest available evidence; discuss the controversies in drug therapy. | |||
| 5 | II | PHARMACOGNOSY | CO1: To understand cell wall constituents and cell inclusion |
| CO2: To understand morphology, microscopy and powder characteristics of crude drugs. | |||
| CO3: To be able to determine the quality of lipids | |||
| CO4: To be able to identify unorganized drugs by chemical methods | |||
| 6 | II | COMMUNITY PHARMACY | CO1: To be able to understand the disease status. |
| CO2:Able to dispense the medication. | |||
| CO3: Able to understand the drug therapy | |||
| CO4: Able to give patient counseling. |
| S.NO | Year/Sem | Course Name | Course Outcomes |
|---|---|---|---|
| 1 | III | PHARMACOLOGY-II | CO1: To study various routes of drug administration, use of anesthetics in laboratory animals and their handling |
| CO2: To learn the composition of physiological salt solutions and basic instruments used in experimental pharmacology | |||
| CO3: To perform isolated experiments using various isolated preparation and the effect of different drugs on the concentration response curves | |||
| CO4: To study the preclinical screening of various drugs | |||
| 2 | III | PHARMACEUTICAL ANALYSIS | CO1: To understand validation of analytical instruments & methods as per ICH/ USP guidelines, concept of quality assurance and quality control techniques. |
| CO2: To understand principles, instrumentation and application of various chromatographic techniques employed for the analysis of APIs and formulation. | |||
| CO3: To understand principle, instrumentation and application of various Electrometric methods | |||
| CO4: To Understand principle, instrumentation and application of UV- Vis, Atomic Absorption and Emission Spectroscopy, Flame Photometry, NMR, Massspectroscopy, Flourimetry, Thermal, X ray diffraction techniques. | |||
| 3 | III | PHARMACOTHERAPEUTICS- II | CO1: To understand therapeutic goals of the drugs used in different diseases |
| CO2: To check & analyze drug interactions, adverse drug reactions | |||
| CO3: To understand dose and frequency of the medications | |||
| 4 | III | PHARMACEUTICAL JURISPRUDENCE | CO1:To appreciate study Pharmaceutical Legislation, relevance and significance of jurisprudence to Pharmaceutical Sciences. |
| CO2: To know fundamentals of legislation to regulate import manufacture, distribution and sales of drug and cosmetics. | |||
| CO3: To know the various parameters in the Drug and Cosmetic Act and rules, Drug policy, Drug Price Control Order, Patent and Design act. | |||
| CO4: To understand the concepts of Narcotic Drugs and Psychotropic substances, Pharmacy Act and Excise duties Act | |||
| 5 | III | MEDICINAL CHEMISTRY | CO1: Understand modern concept of rational drug design. |
| CO2: Learn development of the anti- infective drugs including structure activity relationship, mechanism of action, synthesis, chemical nomenclature, brand names and side effects of important compounds. | |||
| CO3: Understand classification, mechanism of action, structure activity relationship, synthesis, nomenclature and side effects of the drugs acting as antineoplastic agents | |||
| CO4: Understand classification, mechanism of action, structure activity relationship, synthesis, nomenclature and side effects of the drugs acting as Cardiovascular agents, Hypoglycemic agents, Diureties, Steroidal Hormones and Adrenocorticoids etc | |||
| 6 | III | PHARMACEUTICAL FORMULATIONS | CO1: To understand the principle involved in formulation of various pharmaceutical dosage forms |
| CO2: To prepare various pharmaceutical formulation | |||
| CO3: To perform evaluation of pharmaceutical dosage forms | |||
| CO4: To understand and appreciate the concept of bioavailability and bioequivalence, their role in clinical situations |
| S.NO | Year/Sem | Course Name | Course Outcomes |
|---|---|---|---|
| 1 | IV | PHARMACOTHERAPEUTICS | CO1: Initiate drug therapy and the anticipated therapeutic goals by therapeutic intervention |
| CO2: Know the effective use of non- pharmacological therapeutic interventions in the treatment of specific diseases, conditions and symptoms. | |||
| CO3: Demonstrate the ability to effectively communicate and work collaboratively together with others in the small group setting | |||
| CO4: Have moral reasoning, ethical judgement and professionalism | |||
| 2 | IV | HOSPITAL PHARMACY | CO1: To know various drug distribution methods |
| CO2: Know the professional practice management skills ion hospital pharmacies | |||
| CO3: Provide unbiased drug information to the doctors | |||
| CO4: Know The Manufacturing Practices Of Various Formulations In Hospital Set up. | |||
| 3 | IV | CLINICAL PHARMACY | CO1: Monitor drug therapy of patient through medication chart review and clinical review |
| CO2: Obtain medication history interview and counsel the patients. | |||
| CO3: Identify and resolve drug related problems | |||
| CO4: Detect, assess and monitor adverse drug reaction. | |||
| 4 | IV | BIOSTATISTICS & RESEARCH METHODOLOGY | CO1: Know the various statistical Methodology methods to solve different types of problems |
| CO2: Operate various statistical software packages | |||
| CO3: Appreciate the importance of Computer in hospital and Community Pharmacy | |||
| CO4: Appreciate the statistical technique in solving the pharmaceutical problems | |||
| 5 | IV | BIOPHARMACEUTICS & PHARMACOKINETICS | CO1: Broader understanding about the Pharmacokinetics concepts of biopharmaceutics pharmacokinetics. |
| CO2: Ability to calculate various pharmacokinetic parameters by using various mathematical models | |||
| CO3: Ability to design a basic protocol for the conduct of BA/BE study and the interpretation of the BA/BE data | |||
| CO4: Preparedness to use the concepts of pharmacokinetic principles in the clinical contexts. | |||
| 6 | IV | CLINICAL TOXICOLOGY | CO1: Developing general working knowledge of the principles and practice of clinical toxicology |
| CO2: Demonstrating an understanding the health implications of toxic exposures and commonly involved chemicals for toxicity | |||
| CO3: Demonstrating and applying and understanding of general toxicology principles and clinical management practice | |||
| CO4: Demonstrating and applying an understanding of the history, assessment, and therapy considerations associated with the management of a toxic exposure |
| S.NO | Year/Sem | Course Name | Course Outcomes |
|---|---|---|---|
| 1 | V | CLINICAL RESEARCH | CO1: Know the new drug development process. |
| CO2: Understand the regulatory and ethical requirements. | |||
| CO3: Appreciate and conduct the clinical trials activities | |||
| CO4: Know safety monitoring and reporting in clinical trials | |||
| 2 | V | PHARMACOEPIDEMIOLOGY & PHARMACOECONOMICS | CO1: Describe the methods used in Pharmacoepidemiology |
| CO2: Demonstrate competency in the design, conduct and evaluation of Pharmacoepidemiology studies. | |||
| CO3: Describe the methods used in Pharmacoeconomic analysis. | |||
| CO4: Demonstrate competency in the design, conduct and evaluation of Pharmacoeconomic studies. | |||
| 2 | V | CLINICAL PHARMACOKINETICS & PHARMACOTHERAPEUTIC DRUG MONITORINGPHARMACOECONOMICS | CO1: Ability to design a dosage regimen of a drug based on its route of administration |
| CO2: Ability to adjust the dosage regimen for patients with renal impairments | |||
| CO3: Ability to adjust the dosage regimen for patients with hepatic impairments | |||
| CO4: Ability to assess the drug interaction issues in the clinical settings |
Program Educational Outcomes (PEOs),
Program Outcomes (POs), and Program
Specific Outcomes (PSOs) for PharmD (PG
Course)
Program Educational Objectives (PEOs)
PEO 1: Professionalism: Graduates will be able to inculcate leadership & entrepreneurship capabilities and practice ethics & values for providing services to society in promoting healthcare environment & social awareness. Graduates will be able to provide health care information for prevention or treatment of diseases and disorders and demonstrate technical skills in medicine compounding and dispensing of medicines
PEO 2: Academic Excellence and Community wellness:Graduates should be capable of providing high quality pharmaceutical services and striving for excellence in pharmacy education in patent care research & community wellness.
PEO 3: Social Contribution:Graduates should contribute towards health care system by counseling for prophylaxis & prevention of diseases & creating awareness.
PEO 4: Self Identify Awareness: Graduates should update their knowledge & able to analyze pros and cons by organizing or conducting or attending workshops seminars and conferences at national & International Level.
Program Outcomes (POs)
PO 1 Pharmacy Knowledge: Provide high quality, evidence-based, patient-centered care in cooperation with patients, prescribers and members of the inter professional health care team
PO 2 Practical Skill: Demonstrate mastery and application of core knowledge and skills in relation to the evolving biomedical, clinical, epidemiological and social-behavioral sciences.
PO 3 Professional Identity: Evaluate practice and care, and promote continuous improvement in one's own patient care and pharmacy services
PO 4 Problem Solving: Demonstrate self-calibration skills and a commitment to the lifelong learning needed to provide high quality care
PO 5 Communication: Effectively utilize information, informatics and technology to optimize learning and patient care
PO 6 Planning Ability: Demonstrate effective interpersonal written and verbal skills, adapt to socioeconomic and cultural factors as well as situational applications
PO 7 Leadership Skills & Team Work : Demonstrate exemplary professional, ethical and legal behaviors, complying with all federal, state and local laws and regulations related to pharmacy practice
PO 8 Life Long Learning: Demonstrate awareness and responsiveness to the system of healthcare, effectively utilizing systems of care to provide cost-effective, optimal care
PO 9 Pharmaceutical Ethics: Honour personal values and apply ethical principles in professional and social context. Demonstrate behavior that recognizes cultural and personal variability in values, communication and life styles.
PO10 Pharmacist and Society: Apply reasoning informed by the contextual knowledge to asses societal, health, safety and legal issues and the consequent responsibilities relevant to the profession.
PO11 Environment and Society: Understand the impact of professional pharmacy solutions in societal and environmental context and demonstrate the knowledge of, and need for sustainable development.
Program Specific Outcomes (PSOs)
PSO1: Able to apply the knowledge gained during the course of the program in drug discovery and development, their safety and efficacy and current technologies in Pharmaceutical industry
PSO 2: Able to apply the knowledge of ethical and management principles required to work in a team as well as to lead a team.
PSO 3: Able to do multidisciplinary jobs in the pharmaceutical industries and would be able to write effective project reports in multidisciplinary environment in the context of changing technologies.
PHARMACY PRACTICE FACULTY
| S.No | Name of the Faculty | Designation | Qualification |
|---|---|---|---|
| 1 | Dr Manjula Betholi | Professor & H.O.D | M.Pharm, Ph.D |
| 2 | Dr Khaja Zeeyaudddin | Professor | M.Pharm., Ph.D. |
| 3 | M Vasantha | Associate Professor | M.Pharm |
| 4 | Dr Ravinayak | Associate Professor | Pharm.D |
| 5 | Sangeetha Yedla | Assistant Professor | M.Pharm |
| 6 | Srilatha Pagilla | Associate Professor | M.Pharm |
| 7 | Dr Bolla Emmanuel Evangileen | Assistant Professor | Pharm.D |
| 8 | Dr Velpukonda Anudeep | Assistant Professor | Pharm.D |
| 9 | Dr Kunduru Madhuri | Assistant Professor | Pharm.D |
| 10 | Dr Marthi Swathi | Assistant Professor | Pharm.D |
| 11 | Q Tabussum | Assistant Professor | M.Pharm |
| 12 | Dr Tharala Kavya | Assistant Professor | Pharm.D |
Academic Information
Faculty Achivements
STUDENTS ACIEVEMENTS

Student of IV year Pharm.D A.Umika secured 1st place in science crossword BIO-ADHYAYAN 2K25 ~TRENDING NOVEL TECHNOLOGIES IN PHARMACEUTICAL SCIENCES’
Student of III year Pharm.D Abhishek [Rg.No:22GN1TOO21] presented on the topic Advancements in drug delivery systems-Nanotechnology and 3D printing secured 2nd prize in BIO-ADHYAYAN 2K25 –TRENDING NOVEL TECHNOLOGIES IN PHARMACEUTICAL SCIENCES


Student of II year Pharm.D Manisha [Rg.No:23GN1TOO16] secured 1st place in science quiz BIO-ADHYAYAN 2K25 –TRENDING NOVEL TECHNOLOGIES IN PHARMACEUTICAL SCIENCES.
Student of III year Pharm.D M.Sai teja secured 1st prize in oral presentation on the topic Automation and robotics for sorting, packaging and dispensing- EUPHORIA-2K25 conducted by Avanthi Group of Colleges,Gunthapally (V),Abdullapurmet (M),Near Ramoji Filmcity,Hyderabad-501512,Telangana.


AIPS IV Pharm.D students GANTA SURAJ KUMAR [Rg.No:20GN1TOO02] and GUDIPALLI DHARMARAJU [Rg.No:20GN1TOO04] attended & actively participated in Innovation Competition organized by Bhaskar College of pharmacy, Hyderabad.
AIPS IV Pharm D student KURVA CHANDANA [Rg.No:20GN1T0008] attended & actively participated in 73rd Indian pharmaceutical congress conducted at Hitechcity, Exhibition Grounds Hyderabad JULY 5TH, 6TH AND 7TH 2024.


Students of III Pharm.D received certificate for promoting the health and wellbeing of the TSRTC employees across Telangana by participating in TSRTC health screening program-21-05-2024
IV Pharm.D Student Nashra Fatima (Reg No. 20GN1T0020) of Avanthi Institute of Pharmaceutical Sciences got 2nd prize in oral presentation conducted by Sarojini Naidu Vanitha Pharmacy Maha Vidyalaya at Osmania University Hyderabad.


AIPS Student M.SAKETH & V.GAYATRIof PHARM D IV Year received 1st prize for MODEL presentation on the topic ‘ADVANCEMENTS IN HEALTH CARE’ at TWO DAY NATIONAL SEMINAR conducted by AVANTHI Group of Institutions, Hyderabad.
AIPS II Year Pharm.D students attended & actively participated in Awareness program on importance of Clinical Pharmacist in patient care conducted by Gleneagles hospital, Lakdi-ka-pul, Hyderabad.


PHARM.D IV Year students participated in National Conference conducted by Yashoda hospitals, Hitech city, Hyderabad on the topic: ‘‘CLINICAL RESEARCH- TRENDS, TRIALS & TRIBULATION IN INDIAN & GLOBAL SCENARIO’’.
Industrial Visits
II year Pharm.D students visited National Institute of Nutrition located in Hyderabad. Students were actively participated in Industrial Visit and all the students acquired Industrial Knowledge.


III year Pharm.D students Visited Centre for Cellular and Molecular Biology as an industrial visit



Academic Toppers
Pharm. D Academic Toppers

| Name: | G KOYALKAR SHIVANI |
| Roll No | 17GN1T0005 |
| Percentage of Marks | 83.35% |
| Year | 2017-2023 |
| Name: | P BINDHU |
| Roll No | 17GN1T0014 |
| Percentage of Marks: | 82.91% |
| Year | 2017-2023 |


| Name: | G SAI PRAGNA |
| Roll No | 17GN1T0024 |
| Percentage of Marks | 81.78% |
| Year | 2017-2023 |
| Name: | M SHIRISHA |
| Roll No | 18GN1T0012 |
| Percentage of Marks: | 82.15% |
| Year | 2018-2024 |


| Name: | M HIMANGINI |
| Roll No | 18GN1T0008 |
| Percentage of Marks | 81.2% |
| Year | 2018-2024 |
| Name: | B NAVYASRI |
| Roll No | 18GN1T0003 |
| Percentage of Marks: | 79.59% |
| Year | 2018-2024 |

AVANTHI INSTITUTE OF PHARMACEUTICAL SCIENCES (AIPS)
Avanthi Institute of Pharmaceutical Sciences has always been proactive in placing the students into the profiles of their preference. The institute has a well-organized placement team headed by the principal, faculty and the student coordinators.
| Placements for the Academic Year 2023-2024 | ||||
|---|---|---|---|---|
| Sl No. | Name of the student | Branch | Details of the Company | Package |
| 1 | A.ANUSHA | Pharm.D | ASTERPRIME HOSPITAL | 3 LPA |
| 2 | A.CHANDANA | Pharm.D | KIMS HOSPITAL | 3 LPA |
| 3 | NAVYA | Pharm.D | OPTUM MEDICAL CODING | 3.2 LPA |
| 4 | A.SINDHUJHA | Pharm.D | GLOBAL HOSPITAL | 2.6 LPA |
| 5 | D.ANUSHA | Pharm.D | RENOVA NELEMA HOSPITAL | 3 LPA |
| 6 | A.PRAVEEN | Pharm.D | AVIS HOSPITAL | 2.2LPA |
| 7 | HIMANGINI | Pharm.D | SANTOSH HOSPITAL | 2.2 LPA |
| 8 | SAI MANASA | Pharm.D | ASTERPRIME HOSPITAL | 3.2 LPA |
| 9 | MEGHANA | Pharm.D | VEE TECHNOLOGIES PVT LTD 3 | 3.2 LPA |
| 10 | SAMHITHA | Pharm.D | VEE TECHNOLOGIES PVT LTD | 3.2 LPA |
| 11 | SIRISHA | Pharm.D | MEDPLUS HEALTH SERVICES | 3 LPA |
| 12 | ABDUL NAFFEY | Pharm.D | OPTUM GLOBAL SOLUTIONS | 2 LPA |
| 13 | SAI TEJA | Pharm.D | ASTERPRIME HOSPITAL | 3 LPA |
| 14 | VAISHNAVI | Pharm.D | SHADOW HOSPITAL | 2.2 LPA |
| 15 | SUSHMA SWARAJ | Pharm.D | OPTUM GLOBAL SOLUTIONS | 2.2 LPA |
| 16 | SWETHA | Pharm.D | ASTERPRIME HOSPITAL | 3 LPA |
| 17 | PRIYANKA | Pharm.D | OMEGA HOSPITAL | 3 LPA |
| 18 | MEENAKSHI | Pharm.D | YASHODA HOSPITAL | 2 LPA |
| 19 | YOGITHA | Pharm.D | OPTUM GLOBAL SOLUTIONS | 2.2 LPA |
| 20 | PRASANNA | Pharm.D | OMEGA HOSPITAL | 2.5 LPA |
| 21 | SAI ALEKHYA | Pharm.D | OMEGA HOSPITAL | 2.2 LPA |
| 22 | NANDINI | Pharm.D | ABHINAYA HOSPITAL | 2.2 LPA |
| 23 | UMESH | Pharm.D | HETERO DRUGS PRIVATE LTD | 2.2 LPA |
Guest lectures:
Webinar is conducted on IPR on 21-01-2025 by Ministry of Commerce Industry Government of India to all Faculties and to all students
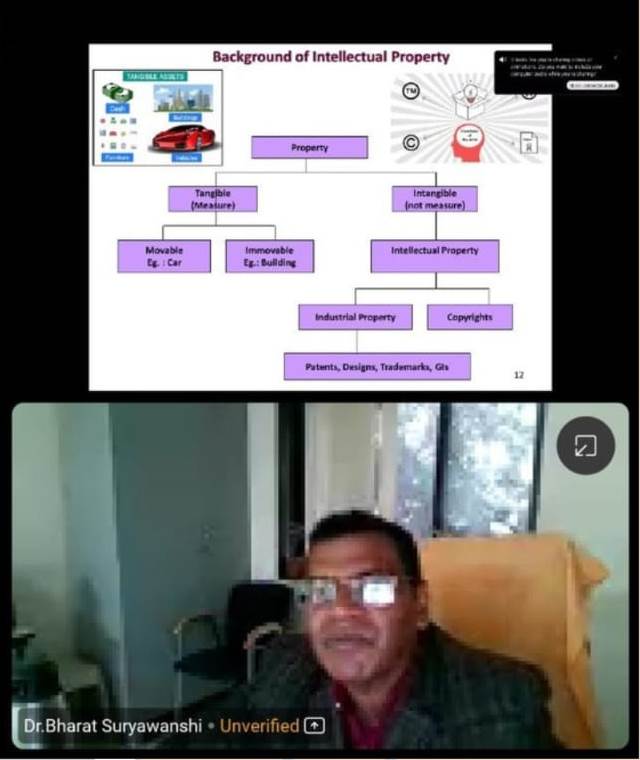
One day seminar on NEP 2020 on 30-01-2025 by Pro Sai Kumar, Motivational speaker for students and faculties

Avanthi Institute of Pharmaceutical Sciences Organized A Guest lecture on the Topic “Multiple Dosage Regimen" delivered by Dr. A. Lalitha Devi, Associate Professor, Department of Pharmacology,G. Pulla Reddy College of Pharmacy, Mehdipatnam, Hyderabad on 25-07-2024. 4th Pharm.D students are actively attended the guest lecture and gained the knowledge on statististical Applications in Health Care and Research.

Guest Lecture on Immunopharmacolog y & Autocoids on 21-12-2024 for Pharm.D II, Pharm.D III & B.Pharmacy III Year students by Dr S Nagarjuna, Pharmacy officer, ESIC MH & PGIMSR, bangalore

Alumni

| Name : | N REVATHI |
| Reg No : | 14GN1T0012 |
| Branch : | Pharm.D |
| Designation : | Senior global Trial specialist |
| Company Name : | Bristol myers squibb |
| Package: | 24 LPA |
| Name : | G HARI KIRAN |
| Reg No : | 14GN1T0005 |
| Branch : | Pharm.D |
| Designation : | Senior global patient recruitment and engagement specialist |
| Company Name : | Bristol myers squibb |
| Package: | 25 LPA |


| Name : | ANISH KUMAR DAS |
| Reg No : | 15GN1T0019 |
| Branch : | Pharm.D |
| Designation : | Junior clinical research associate |
| Company Name : | Novo nordisk |
| Package: | 5.6 LPA |
| Name : | A CHANDANA |
| Reg No : | 16GN1T0001 |
| Branch : | Pharm.D |
| Designation : | Clinical pharmacist |
| Company Name : | Yashoda hospitals |
| Package: | 4.2 LPA |


| Name : | D SINDHU |
| Reg No : | 17GN1T0015 |
| Branch : | Pharm.D |
| Designation : | Clinical research coordinator |
| Company Name : | Novonordisk |
| Package: | 5.4 LPA |
| Name : | A SINDHU |
| Reg No : | 16GN1T0021 |
| Branch : | Pharm.D |
| Designation : | Executive medical affairs |
| Company Name : | Pulse pharmaceuticals |
| Package: | 4 LPA |

Gallery
FRESHERS DAY CELEBRATION- UDBHAV 2K24-25






TRADITIONAL DAY CELEBRATIONS-2024


INTERNATIONAL YOGA DAY-2024

FAREWELL DAY-2024

SIGNATURE DAY
MEDICAL CAMP

CAMPUS PLACEMENT DIVE-2023







